Plank's Blackbody Distribution Law
The intensity of radiation emitted from an object is a function of its temperature, wavelength, and emissivity. A perfect emitter, also known as a blackbody, is a material that radiates 100% of the electromagnetic energy that is theoretically possible for a material at a specified temperature. Planck’s blackbody equation, developed by Max Planck in 1901, defines the intensity of radiation for a blackbody as a function of temperature and wavelength.
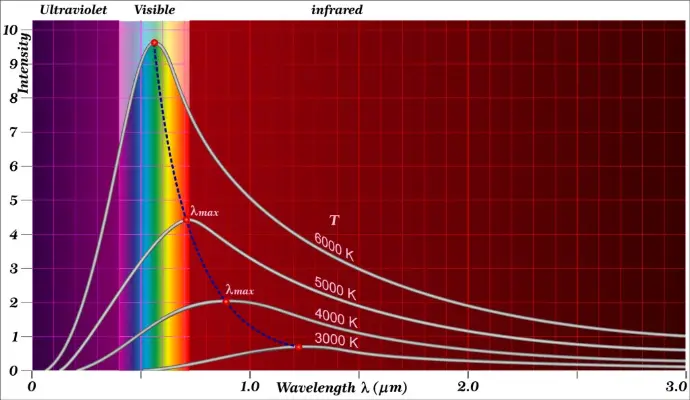
Each plot in Planck’s blackbody curve represents the energy distribution at a different temperature. As temperature increases, the intensity of radiation increases. Additionally, as the temperature of an object increases, the peak intensity moves to shorter wavelengths. The surface of the sun, at 6000°C, has its peak in the yellow region of the visible portion of the spectrum, and therefore, appears yellow.
We can also observe this phenomenon by slowly heating a piece of metal. At room temperature, the metal does not emit any light visible to the human eye. However, if we heat it to approximately 500°C, it begins to glow red. At 500°C, the metal emits visible light primarily in the red portion of the visible spectrum. As the temperature of the metal increases to approximately 1500°C, it begins to glow white. At 1500°C, the metal emits energy at all visible wavelengths. White light is the combination of all visible colors.
Objects that emit energy well also absorb energy well. Therefore, a perfect emitter is also a perfect absorber. The term blackbody arose because a perfect absorber absorbs all visible electromagnetic energy (light) and appears black. Blackbodies are useful as a reference to characterize the behavior of materials. The relationship between the intensity of energy emitted by a blackbody at various wavelengths as a function of temperature was discovered by Max Planck in 1900.
There are no comments for now.